Edit
Delete
Lemon Battery
The Adventure
Investigate chemical reactions and electricity and create a battery from a simple piece of fruit.
Plan
- Investigate electricity - what is it, how does it work, what is required for an electrical current, electrical circuits, and conductivity?
- Investigate the key properties of lemons, copper metal, and zinc metal and their relationship to conductivity.
- Read the safety information and discuss with your leaders or another appropriate adult what safety equipment, precautions, and supervision may be required. Ensure that you have these safety measures in place before starting the ‘Do’ section.
- Gather all the equipment you need to make your lemon battery. You will need a lemon, a copper strip, a zinc strip, a knife, 2 copper wire leads (about 20 cm long each) with alligator clips on both ends, an LED bulb with a rating of no more than 2 volts (in this case, the lower the voltage, the better), wire cutters, and wire strippers. Whilst not essential, it is very useful to have a multimeter available.
Do
- Release some of the lemon juices into the lemon by firmly rolling the lemon on a table or other hard surface.
- Insert the copper strip into the lemon with one end sticking out.
- Insert the zinc strip into the lemon with one end sticking out.
- Connect a copper wire lead to each metal (copper and zinc) strip with the alligator clip.
- Connect one of the free ends of the wire leads to one of the wires attached to the LED bulb.
- Connect the remaining free wire lead end to the remaining free wire on the LED bulb. Your lemon battery should look similar to the image below.
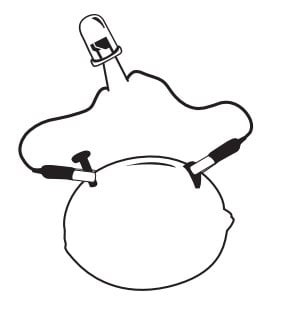
Image Source: https://www.scienceworld.ca/resource/lemon-battery/
- If you have a multimeter, check the voltage between the two electrodes. It will likely be quite low and may not be enough to power the LED light. You should also make sure that your LED light is connected in the correct direction for the light to glow.
- If the light does not glow, try forming a circuit with multiple lemons and connecting the copper electrode to the zinc electrode. How many lemons do you need to make the light glow?
Review
- Did you manage to make the light glow? If not, what do you think went wrong?
- What do you think you could do differently next time to improve your lemon battery?
- Do you think the battery would still work with other fruits or vegetables? Why or why not? Which fruit or vegetable do you think would work best?
Safety
- Whilst this challenge card experiments with electricity, it is a very low voltage.
- Do not clip alligator clips on fingers, skin, or other body parts as they may present a pinch risk.
- Lemons and lemon juice are high in citric acid. Once you have pierced the lemon, be careful to avoid any lemon juice getting in eyes or open wounds as this may irritate them. If contact does occur, wash thoroughly with clean water.
Variations
- A galvanised nail (zinc coated) can work as a good substitute for the zinc strip.
- Experiment with different variables such as different metals for the electrodes and different fruits and vegetables. Challenge your patrol to see who can produce the highest voltage.
- Experiment with what happens if you use the same type of metal for each electrode.
- See if you can power something else.
- Add an environmental twist to this Challenge Card with discussion about different and renewable sources of electricity.
























